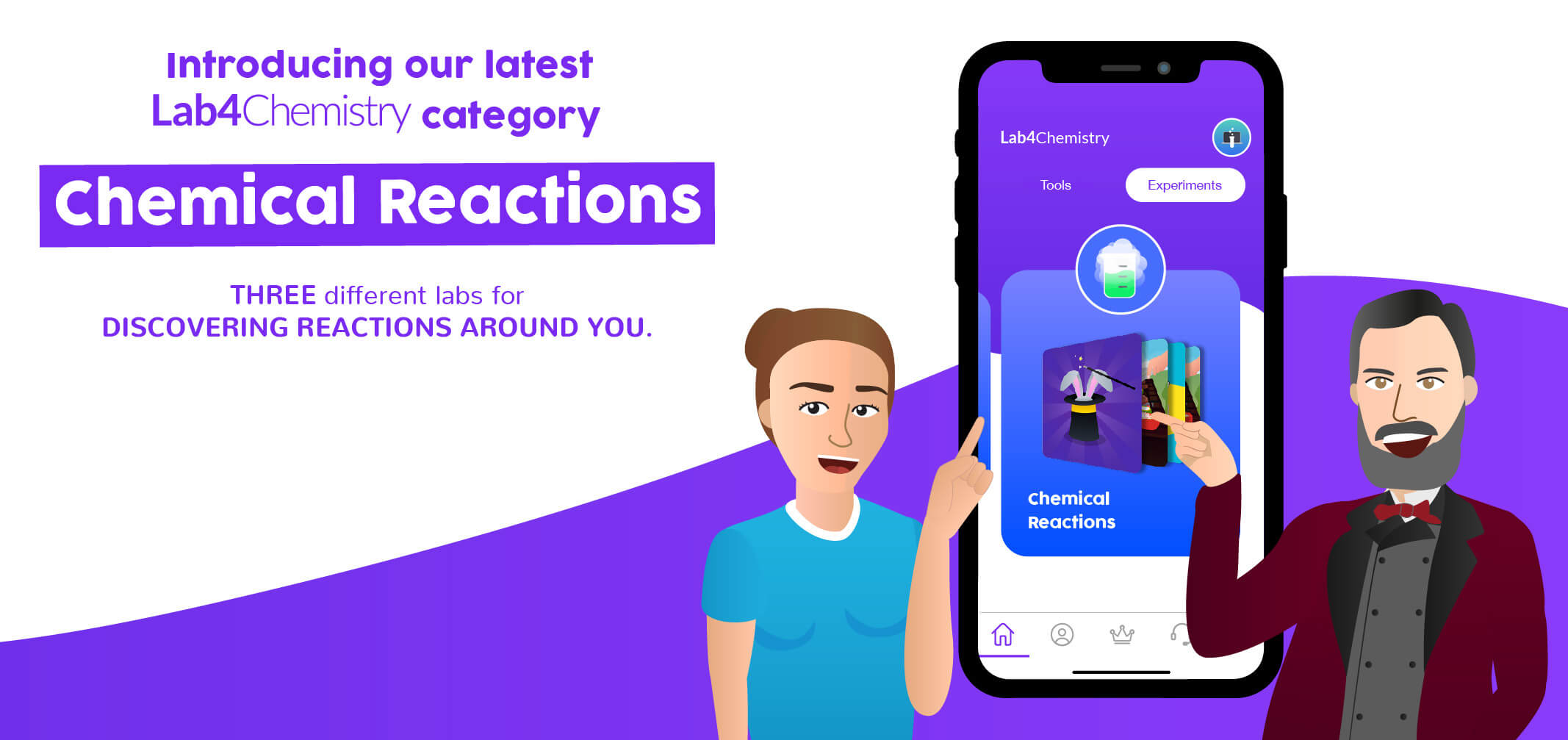
Lab4Chemistry’s brand new category 😱 Chemical Reactions!
By Lab4U Team

Food decomposition, glucose formation in plants, engine combustion, metals oxidizing⏤these are just some of the countless examples of chemical reactions we encounter every day. Technically speaking, a chemical reaction involves the transformation of one substance into another. During the conversion process, bonds may form or break and molecules may rearrange, but no atom is created or destroyed.
This concept is central to our understanding of the world. It has allowed scientists to better comprehend the behavior of matter, which has resulted in substantial leaps forward in technological and industrial development in recent centuries. The food, cosmetic, pharmacological, mining, and energy industries, among others, all owe a great deal to chemistry.
We also should acknowledge chemistry’s significant contribution to environmental causes as well as economic ones. For instance, the behavior of chlorofluorocarbons (CFCs), their interaction with the ozone layer, and the effects of greenhouse gases are all phenomena better understood through the lens of chemical reactions.
A third, equally important branch of study is biochemistry. By investigating how chemical reactions work within living beings, we have been able to make impressive breakthroughs in medicine by harnessing the power of plant, chemical, and other material resources.
Of course, chemical reactions can also be fascinating and illuminating on an instructional level. Learning about chemical processes is a good entry point for analyzing Socio-scientific Issues (SSI), studying the Nature of Science and the History of Science (NOS and HOS), and promoting experimentation within investigative experiences.
The team at Lab4U has been similarly inspired by chemical reactions. As a result, we have developed three categories to help teachers and students further their studies with hands-on experiences. The first is a general category, which introduces phenomena that involve chemical changes and the stoichiometric relationships that form between reactants and products. The second and third are more specific, covering the physicochemical parameters of chemical reactions and acid-base balances (we’ll discuss them in more detail in our next Blog).

As of now, we have three engaging experiences for students in our chemical reactions category. In the first, they generate chemical reactions and observe the ways in which a reaction manifests itself (including the production of light, changes in the temperature of the system, effervescence, formation of a precipitate, color change, or release of a gas). Along the way, students will also get a feel for methodology and processes, a fundamental skill in the study of chemistry.
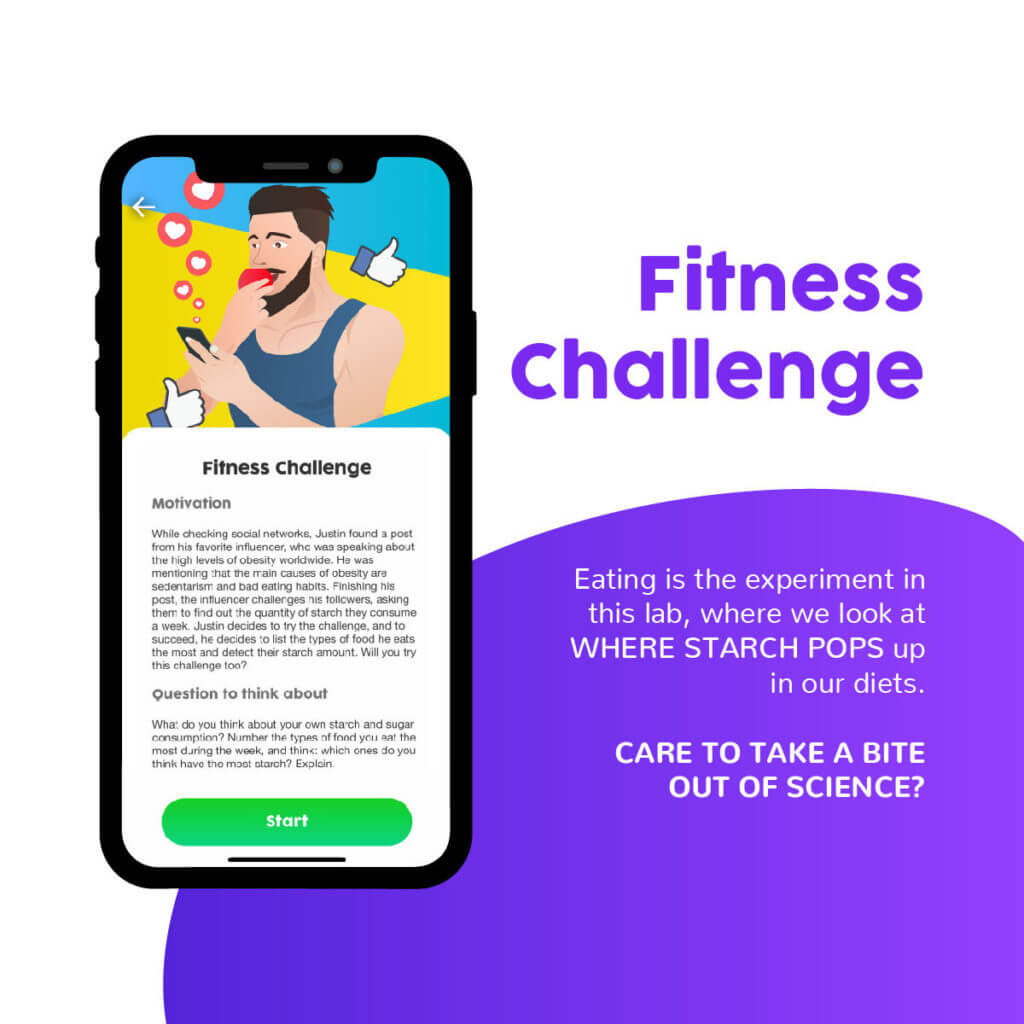
The second experience explores how the study of a chemical reaction can impact a person’s lifestyle. By examining the consumption of starch in their diets, students will learn about how scientific knowledge can help them make better informed health decisions. These types of objectives are part of the third level of critical scientific literacy proposed by Sjöström (2014). In his article, he explains that a body of scientific knowledge is at its highest level, the emancipatory critical focus, when it helps citizens develop critical, reflective thinking skills and make informed decisions on social and personal issues.
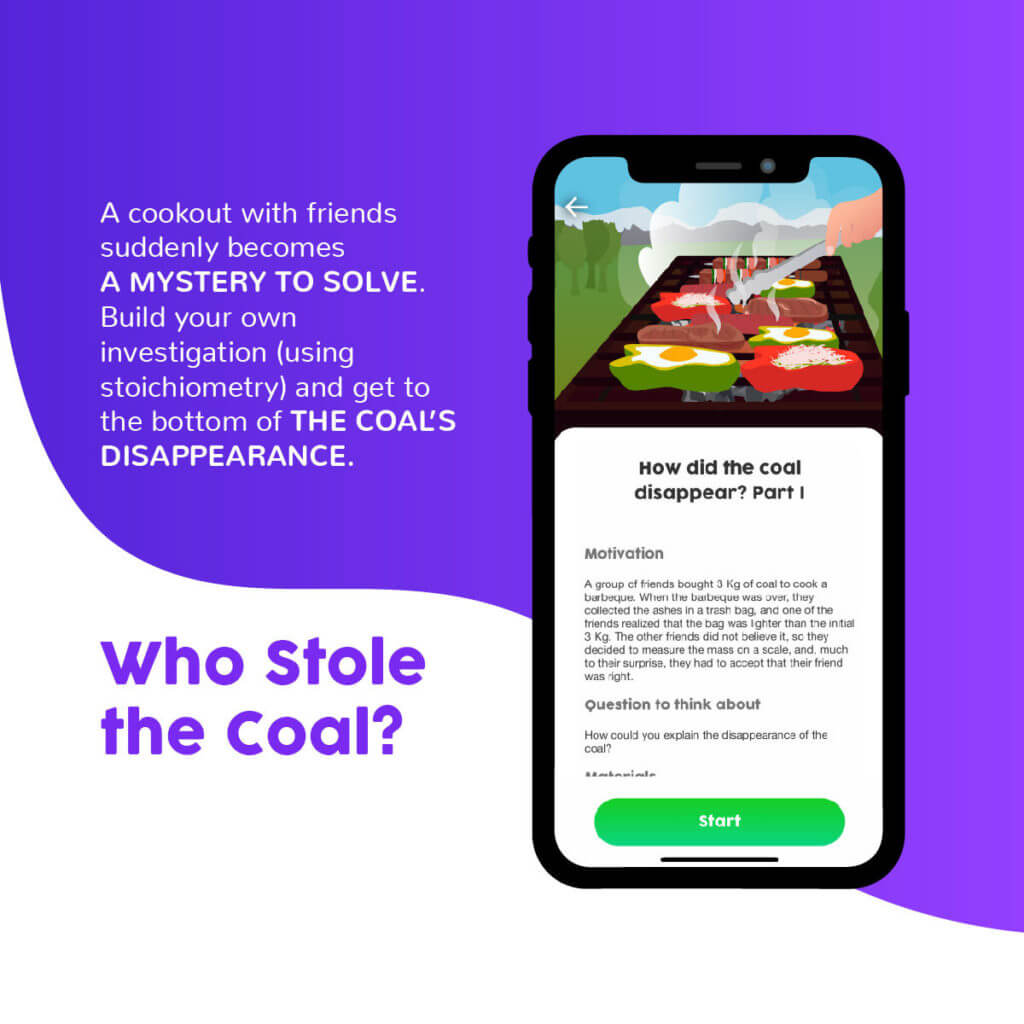
The third experience is a small stoichiometry research project, where students are presented with a mystery they must solve. It isbased on a semi-structured inquiry (Vergara & Cofré, 2012), where a problem is posed and it is up to the students to design an experiment to address it. Therefore, it can be a helpful tool for exploring the Nature of Sciences (NOS) in parallel, as there is no single “scientific method.”
We invite all our readers to dig into the new Lab4U experiences and experiment with Lab4Chemistry. The next Einstein or Marie Curie could be at your school!
References
Sjöstrom, J; Stenborg, E. (2014) Science education research and education for sustainable development, 37-46, Shaker, Aachen, Germany
Vergara, C., Cofré, H. L. (2012). La indagación científica: un concepto esquivo, pero necesario. Revista chilena de educación científica, 11(1), 30-38.




